Perjeta (Pertuzumab) 420nmg/14 ml Imported Available In Pakistan
Perjeta (pertuzumab) is a targeted therapy used primarily for the treatment of HER2-positive breast cancer. It is available as a concentrate for solution for infusion, specifically in a 420 mg/14 mL formulation. Here are key details regarding its availability and usage in Pakistan:
Product Overview
- Name: Perjeta
- Active Ingredient: Pertuzumab
- Formulation: Concentrate for solution for infusion
- Strength: 420 mg per 14 mL vial
- Concentration: 30 mg/mL
Indications
Perjeta is indicated for use in combination with trastuzumab and docetaxel for the treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease
Original price was: ₨40,000.00.₨36,000.00Current price is: ₨36,000.00.
Description
Perjeta (pertuzumab) is a targeted therapy used primarily for the treatment of HER2-positive breast cancer. It is available as a concentrate for solution for infusion, specifically in a 420 mg/14 mL formulation. Here are key details regarding its availability and usage in Pakistan:
Product Overview
- Name: Perjeta
- Active Ingredient: Pertuzumab
- Formulation: Concentrate for solution for infusion
- Strength: 420 mg per 14 mL vial
- Concentration: 30 mg/mL
Indications
Perjeta is indicated for use in combination with trastuzumab and docetaxel for the treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease
Key Benefits
- Targeted Therapy: Perjeta specifically targets the HER2 protein, inhibiting its dimerization with other HER family members, which is crucial for tumor growth in HER2-positive cancers
- Improved Survival Rates: Clinical studies, such as the CLEOPATRA trial, have demonstrated that Perjeta, when used in combination with trastuzumab and docetaxel, significantly improves progression-free survival and overall survival in patients with HER2-positive metastatic breast cancer
- Dual Mechanism of Action: When combined with trastuzumab, Perjeta provides a dual blockade of HER2-driven signaling pathways, enhancing the therapeutic effect beyond what either drug could achieve alone
- Use in Various Treatment Settings: Perjeta is indicated for both metastatic and early-stage HER2-positive breast cancer, including neoadjuvant and adjuvant settings
Key Ingredients
- Active Ingredient: The primary active ingredient in Perjeta is pertuzumab, a humanized IgG1 monoclonal antibody.
- Concentration: Each 14 mL vial contains 420 mg of pertuzumab, resulting in a concentration of 30 mg/mL
- Production Method: Pertuzumab is produced using recombinant DNA technology in mammalian (Chinese hamster ovary) cells
- Excipients: While the specific excipients are not detailed in the summary, they typically include substances necessary to stabilize the formulation for intravenous use.
- Perjeta (pertuzumab) is an effective treatment for HER2-positive breast cancer, working through a specific mechanism to inhibit cancer cell growth. Here’s how it functions:
Mechanism of Action
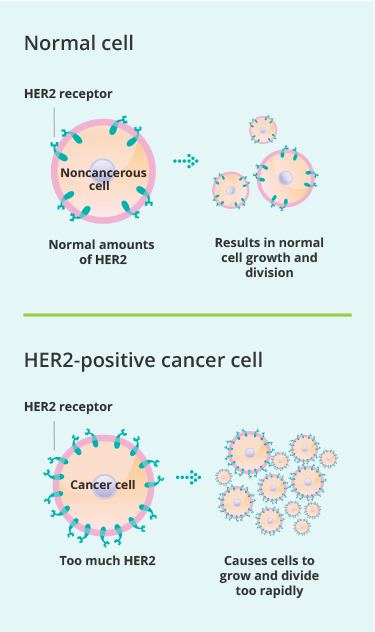
Targeting HER2 Protein: Perjeta specifically binds to the HER2 protein, which is overexpressed in some breast cancer cells. This protein is part of the human epidermal growth factor receptor family, and its overexpression leads to increased cell growth and division, contributing to cancer progression
.Blocking Dimerization: By binding to a specific region known as subdomain II of the HER2 receptor, Perjeta prevents HER2 from forming heterodimers with other receptors, such as HER3 and HER4. This dimerization is crucial for activating downstream signaling pathways that promote cell proliferation and survival
. Thus, Perjeta effectively disrupts these signaling pathways, leading to reduced cancer cell growth.Inducing Immune Response: In addition to blocking growth signals, Perjeta enhances the immune system’s ability to target cancer cells. It mediates a process called antibody-dependent cellular cytotoxicity (ADCC), where immune cells are recruited to attack and kill the cancer cells marked by Perjeta
Combination Therapy
Perjeta is often used in combination with trastuzumab (Herceptin) and chemotherapy. While both drugs target HER2, they bind to different sites on the receptor, providing a dual blockade of HER2 signaling pathways. This combination is more effective than using either drug alone, significantly improving treatment outcomes for patients with HER2-positive breast cancer.
- Perjeta (pertuzumab) is administered as an intravenous (IV) infusion, and its administration process is structured to ensure safety and efficacy. Here’s a detailed overview of how Perjeta is administered during treatment:
Administration Process
- Initial Dose:
- The initial loading dose of Perjeta is 840 mg, which is administered as a 60-minute intravenous infusion. This initial infusion allows healthcare providers to monitor the patient’s response and manage any potential infusion-related reactions effectively.
- Subsequent Doses:
- After the initial dose, maintenance doses of 420 mg are given every three weeks. These subsequent infusions can be administered over a shorter duration of 30 to 60 minutes, depending on the patient’s tolerance.
- Combination Therapy:
- Perjeta is typically administered in combination with trastuzumab (Herceptin) and docetaxel. The administration sequence can vary, but generally, Perjeta and trastuzumab can be given in any order, followed by docetaxel.
- For trastuzumab, the initial dose is 8 mg/kg, followed by a maintenance dose of 6 mg/kg every three weeks.
- Docetaxel is usually started at a dose of 75 mg/m², with adjustments based on tolerability.
- Observation Period:
- After each Perjeta infusion, an observation period of 30 to 60 minutes is recommended to monitor for any adverse reactions before proceeding with subsequent infusions of trastuzumab or docetaxel.
- Administration Settings:
- Perjeta should only be administered by healthcare professionals experienced in cancer treatment, and it must not be given as an IV push or bolus.
- Re-administration Guidelines:
- If the time between two sequential infusions exceeds six weeks, the initial loading dose of 840 mg must be re-administered before resuming the maintenance schedule.
- Dietary restrictions during cancer treatment are essential for managing side effects and supporting overall health. Here’s a summary of key dietary guidelines, including foods to add and avoid:
Foods to Add
- High-Protein Foods: Essential for tissue repair and maintaining strength. Include:
- Lean meats (chicken, turkey, fish)
- Eggs
- Low-fat dairy (Greek yogurt, milk, cheese)
- Plant-based proteins (beans, legumes, nuts)
- Healthy Fats: Important for calorie intake and reducing inflammation. Sources include:
- Fatty fish (salmon, mackerel)
- Avocados
- Olive oil
- Nuts and seeds
- Fruits and Vegetables: Rich in vitamins, minerals, and antioxidants. Aim for a variety to maximize nutrient intake:
- Dark leafy greens
- Berries
- Citrus fruits
- Whole Grains: Provide fiber and essential nutrients. Choose:
- Brown rice
- Quinoa
- Whole grain bread and pasta
- Hydration: Staying hydrated is crucial; consider electrolyte-enhanced drinks if experiencing diarrhea or vomiting.
Foods to Avoid
- Raw or Undercooked Foods: Due to compromised immunity, avoid:
- Raw fish (sushi)
- Undercooked eggs and meats
- Unpasteurized dairy products
- Processed Foods: Limit intake of heavily processed or packaged foods that lack nutritional value:
- Sugary snacks and beverages
- Fried foods containing hydrogenated oils
- High-Sugar Foods: While sugar doesn’t directly “feed” cancer, reducing sugar intake can benefit overall health.
- Spicy or Acidic Foods: These can irritate the digestive system or mouth sores common during treatment.
- High-Fiber Foods: In some cases, especially if experiencing diarrhea, it may be necessary to limit high-fiber foods temporarily.
- Caffeinated Beverages and Alcohol: These can exacerbate dehydration and interfere with treatment.
Special Considerations
- Small Meals: Eating smaller, more frequent meals can help manage appetite loss and nausea.
- Soft Foods: If experiencing difficulty swallowing or mouth sores, opt for soft foods like smoothies, yogurt, and pureed meals.
- Consultation with Dietitians: It’s advisable for cancer patients to work with registered dietitians who can tailor dietary plans based on individual treatment regimens and side effects.

Key Precautions
- Cardiac Monitoring:
- Patients should undergo regular cardiac assessments, including monitoring left ventricular function, as Perjeta can cause heart problems such as left ventricular dysfunction and congestive heart failure. This is particularly important for those with a history of heart disease or who have previously received anthracycline treatments
- Pregnancy and Breastfeeding:
- Perjeta is contraindicated during pregnancy due to the risk of fetal harm, including potential birth defects and embryo death. Women of childbearing age should use effective contraception during treatment and for at least 6 months after the last dose. It is also not recommended for breastfeeding mothers
- Allergic Reactions:
- Patients should be monitored for signs of allergic reactions, which can include symptoms such as difficulty breathing, facial swelling, or rash. Any severe allergic reactions may necessitate stopping the infusion and providing appropriate medical intervention
- Infection Risk:
- Perjeta can lower white blood cell counts (neutropenia), increasing the risk of infections. Patients should be vigilant about signs of infection and report any symptoms such as fever or chills to their healthcare provider immediately
- Live Vaccines:
- Patients receiving Perjeta should avoid live vaccines, as their immune response may be compromised, potentially leading to ineffective vaccination or increased risk of infection.
- Surgery Considerations:
- Inform healthcare providers about Perjeta treatment prior to any surgical procedures, as adjustments may be necessary based on treatment timing and patient health status
- Drug Interactions:
- Patients must disclose all medications they are taking, including over-the-counter drugs and herbal supplements, to prevent potential interactions with Perjeta
- Side Effects Management:
- Common side effects include diarrhea, nausea, fatigue, hair loss, and skin rashes. Patients should discuss any side effects with their healthcare team to manage them effectively and adjust treatment if necessary
Drug Interactions
- No Significant Pharmacokinetic Interactions:
- Clinical studies have shown no significant pharmacokinetic (PK) interactions between Perjeta and commonly used agents in HER2-positive breast cancer treatment, such as trastuzumab and docetaxel. This means that the presence of Perjeta does not significantly alter the absorption, distribution, metabolism, or excretion of these drugs
- Cytotoxic Agents:
- Studies evaluating the effects of Perjeta on other cytotoxic agents, including gemcitabine, erlotinib, and capecitabine, also indicated no evidence of drug-drug interactions. The pharmacokinetics of Perjeta remained comparable to those observed in single-agent studies
- Anthracyclines:
- There is insufficient evidence to recommend the concomitant use of anthracyclines (like doxorubicin) with Perjeta. While no direct interaction has been established, caution is advised due to the potential for increased cardiac toxicity when combining these agents
- Monitoring for Side Effects:
- Although there are no significant pharmacokinetic interactions, patients receiving Perjeta in combination with other therapies should be monitored for cumulative side effects, particularly those related to cardiac function and bone marrow suppression (e.g., neutropenia)
- Infusion Reactions:
- Patients may experience infusion-related reactions when receiving Perjeta, especially if administered on the same day as other treatments. Common reactions include fatigue, fever, chills, and hypersensitivity . It is recommended to observe patients for 30 to 60 minutes post-infusion
Medications to Avoid
- Anthracyclines:
- Doxorubicin (Adriamycin)
- Daunorubicin
- Epirubicin
- Idarubicin
- Pirarubicin
- Pixantrone
- Valrubicin
These medications are associated with increased risk of cardiac toxicity when used in conjunction with Perjeta. There is insufficient evidence to recommend their concurrent use, and caution is advised due to the potential for exacerbating heart-related side effects, particularly in patients with pre-existing heart conditions or those who have received prior anthracycline therapy
live vaccine- Patients receiving Perjeta should avoid live vaccines because their immune response may be compromised, making vaccination less effective and increasing the risk of vaccine-related infections
.
- Other Immunosuppressive Agents:
- While specific interactions with other immunosuppressive drugs are not well documented, caution is advised when combining Perjeta with other agents that may further suppress the immune system.
Dosage
- Initial Dose: The recommended initial dose of Perjeta is 840 mg, administered as an intravenous (IV) infusion over 60 minutes.
- Maintenance Dose: Following the initial dose, Perjeta is given every three weeks at a dosage of 420 mg, administered as an IV infusion over 30 to 60 minutes.
- Combination Therapy: Perjeta is typically used in combination with trastuzumab and docetaxel. The initial dose of trastuzumab is 8 mg/kg, followed by 6 mg/kg every three weeks. Docetaxel is usually started at 75 mg/m², with possible escalation to 100 mg/m² based on tolerance.
Storage
- Perjeta should be stored in the refrigerator at a temperature between 2°C to 8°C (36°F to 46°F).
- It must be protected from light and should not be frozen.
- Once removed from refrigeration, it can be stored at room temperature (up to 25°C or 77°F) for a maximum of 24 hours before use.
- Any unused portion of the vial should be discarded after a single use.
Reviews
Efficacy
- Clinical trials have demonstrated that Perjeta effectively delays disease progression and improves overall survival rates in patients with HER2-positive breast cancer. It is particularly beneficial in metastatic cases and as neoadjuvant therapy before surgery.
- Cardiac Monitoring:
- High-Protein Foods: Essential for tissue repair and maintaining strength. Include:
- Initial Dose:






Reviews
There are no reviews yet.