Valtrex 500mg 10’ct tablet
Valtrex 500mg is a prescription antiviral medication used to treat certain viral infections caused by the herpes simplex virus (HSV) and varicella-zoster virus (VZV). The 500mg strength comes in a white, biconvex, elongated film-coated tablet with a white to off-white core, engraved “GX CF1” on one side.Some of the key uses of Valtrex 500mg include:
- Treatment of herpes zoster (shingles) and ophthalmic zoster in immunocompetent adults
- Treatment of first-episode and recurrent genital herpes in immunocompetent adults and adolescents
- Suppression of recurrent genital herpes in immunocompetent adults and adolescents
- Treatment of herpes labialis (cold sores) in adults and adolescents
The typical adult dosage for most indications is 500mg taken orally twice daily. For herpes labialis, the dose is 2000mg taken twice daily for one day. The dosage may need to be adjusted in patients with impaired renal function.Common side effects include headache, nausea, abdominal pain, neutropenia, and elevated liver enzymes. Valacyclovir is the prodrug of acyclovir, which inhibits replication of herpes viral DNA.
>IMPORTENT NOTE… Those customers who belongs to lahore avail the opportunity of cash on delivery(COD) with in 30mints.
> Available on cheap price
> ALL ALTERNATIVES BRANDS ARE AVAILABLE
>ORIGNAL & IMPORTED
> For maore details & price kindly contact on whatsapp.
Description
Valtrex 500mg is a prescription antiviral medication used to treat certain viral infections caused by the herpes simplex virus (HSV) and varicella-zoster virus (VZV). The 500mg strength comes in a white, biconvex, elongated film-coated tablet with a white to off-white core, engraved “GX CF1” on one side.Some of the key uses of Valtrex 500mg include:
- Treatment of herpes zoster (shingles) and ophthalmic zoster in immunocompetent adults
- Treatment of first-episode and recurrent genital herpes in immunocompetent adults and adolescents
- Suppression of recurrent genital herpes in immunocompetent adults and adolescents
- Treatment of herpes labialis (cold sores) in adults and adolescents
The typical adult dosage for most indications is 500mg taken orally twice daily. For herpes labialis, the dose is 2000mg taken twice daily for one day. The dosage may need to be adjusted in patients with impaired renal function.Common side effects include headache, nausea, abdominal pain, neutropenia, and elevated liver enzymes. Valacyclovir is the prodrug of acyclovir, which inhibits replication of herpes viral DNA.
Medication Overview
- Valtrex 500mg is a prescription antiviral medication used to treat certain viral infections caused by the herpes simplex virus (HSV) and varicella-zoster virus (VZV).
- The 500mg strength comes in a white, biconvex, elongated film-coated tablet with a white to off-white core, engraved “GX CF1” on one side.
Approved Uses
- Treatment of herpes zoster (shingles) and ophthalmic zoster in immunocompetent adults
- Treatment of first-episode and recurrent genital herpes in immunocompetent adults and adolescents
- Suppression of recurrent genital herpes in immunocompetent adults and adolescents
- Treatment of herpes labialis (cold sores) in adults and adolescents
Dosing Information
- The typical adult dosage for most indications is 500mg taken orally twice daily.
- For herpes labialis, the dose is 2000mg taken twice daily for one day.
- The dosage may need to be adjusted in patients with impaired renal function.
Side Effects
- Common side effects include headache, nausea, abdominal pain, neutropenia, and elevated liver enzymes.
- Valacyclovir is the prodrug of acyclovir, which inhibits replication of herpes viral DNA.
Key Benefits:
- Accelerates the resolution of pain associated with herpes zoster (shingles)
- Reduces the duration and proportion of patients with zoster-associated pain
- Effective treatment for first-episode and recurrent genital herpes in immunocompetent adults and adolescents
- Suppresses recurrent genital herpes in immunocompetent adults and adolescents
- Treats herpes labialis (cold sores) in adults and adolescents
Key Uses:
- Treatment of herpes zoster (shingles) and ophthalmic zoster in immunocompetent adults
- Treatment of herpes zoster in adult patients with mild or moderate immunosuppression
- Treatment of first-episode and recurrent genital herpes in immunocompetent adults and adolescents
- Suppression of recurrent genital herpes in immunocompetent adults and adolescents
- Treatment of herpes labialis (cold sores) in adults and adolescents
Precautions for Valtrex in Patients with Kidney Disease
Acute Renal Failure
- Cases of acute renal failure have been reported in elderly patients with or without reduced renal function. Caution should be exercised when administering Valtrex to geriatric patients, and dosage reduction is recommended for those with impaired renal function.
Dosage Reduction Recommended
- Dosage reduction is recommended when administering Valtrex to patients with renal impairment.
Avoid High Doses in Patients with Underlying Renal Disease
- Patients with underlying renal disease who received higher-than-recommended doses of Valtrex for their level of renal function are at risk. Dosage reduction is recommended when administering Valtrex to patients with renal impairment.
Importance of Hydration
- Adequate hydration should be maintained for all patients taking Valtrex, as precipitation of acyclovir in renal tubules may occur when the solubility is exceeded in the intratubular fluid.
Hemodialysis for Acute Renal Failure
- In the event of acute renal failure and anuria, the patient may benefit from hemodialysis until renal function is restored.
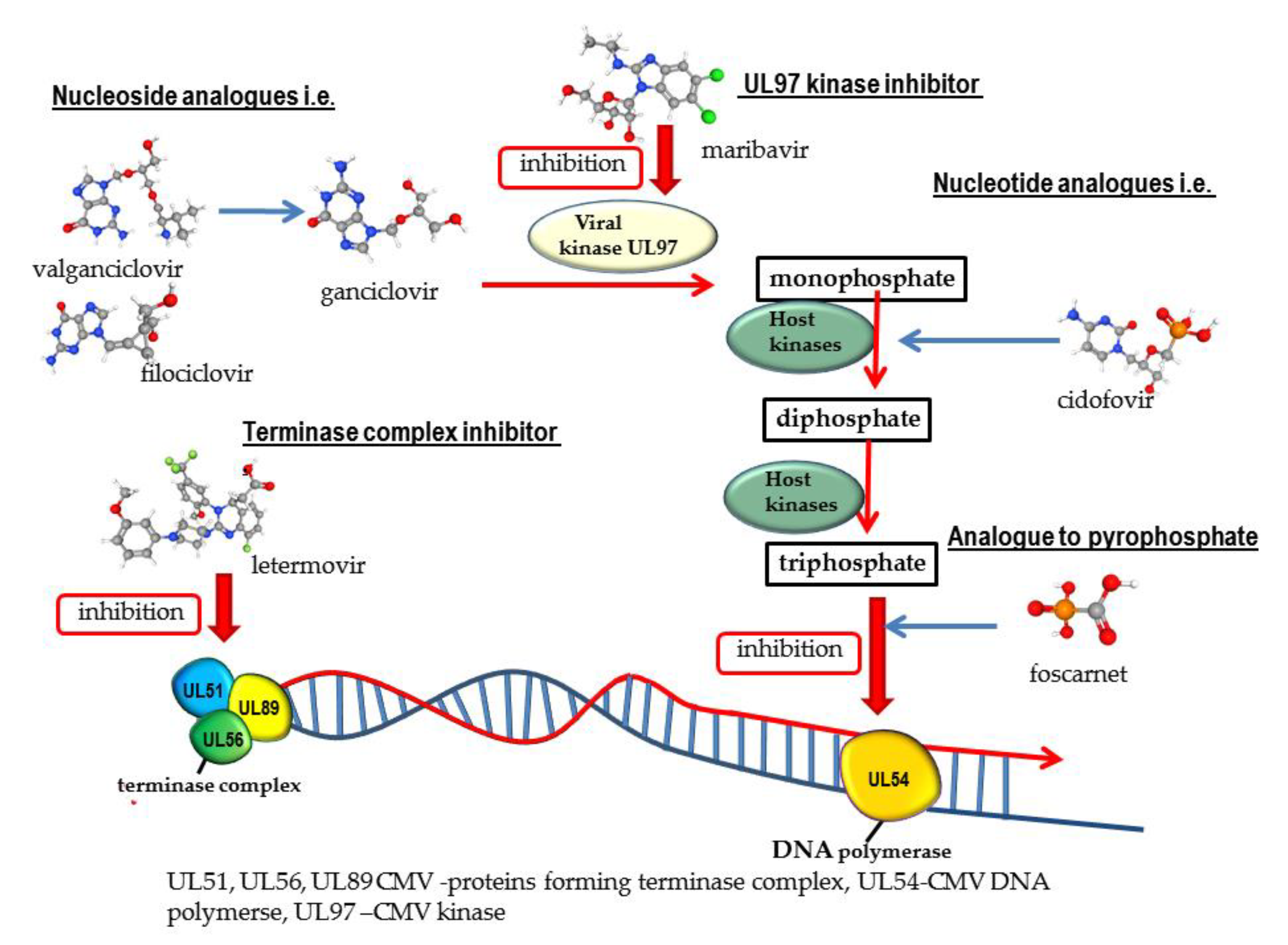
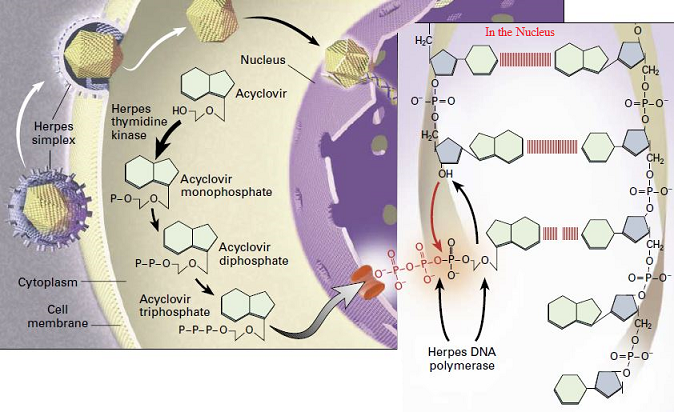
Mechanism of Action of Valtrex (Valacyclovir)
Valtrex (valacyclovir) is a prodrug that is converted to the active antiviral agent acyclovir in the body. The key aspects of Valtrex’s mechanism of action are:Conversion to Acyclovir
- Valacyclovir is rapidly converted to acyclovir, the active antiviral compound, after oral administration.
- This conversion occurs through the action of intestinal and hepatic enzymes that hydrolyze the valyl ester.
Inhibition of Viral DNA Synthesis
- Once converted to acyclovir, it is further phosphorylated by viral and cellular kinases to the active acyclovir triphosphate.
- Acyclovir triphosphate inhibits viral DNA synthesis through several mechanisms:
- Competitive inhibition of viral DNA polymerase
- Incorporation into and termination of the growing viral DNA chain
- Inactivation of the viral DNA polymerase
Selective Activation in Infected Cells
- The initial phosphorylation step that converts acyclovir to its active form is catalyzed more efficiently in virus-infected cells compared to uninfected cells.
- This selective activation in infected cells contributes to the antiviral specificity of acyclovir.
Valtrex Use During Pregnancy
- Valtrex should be used during pregnancy only if the potential benefits outweigh the potential risks. There are no controlled data on Valtrex use in human pregnancy.
- Animal studies have not shown evidence of embryofetal toxicity or teratogenicity with Valtrex or its active metabolite acyclovir at exposures up to 4-7 times the maximum recommended human dose.
- However, there is a risk of neonatal herpes simplex virus (HSV) infection, which varies from 30-50% for genital HSV acquired in late pregnancy. A primary HSV occurrence in early pregnancy has been associated with rare cases of congenital infection.
- The Acyclovir and Valacyclovir Pregnancy Registries have collected data on pregnancy outcomes, but the sample size is not large enough to definitively evaluate the safety of Valtrex during pregnancy.
- Overall, Valtrex may be used during pregnancy if the potential benefits outweigh the potential risks, especially for suppressing recurrent genital herpes near term to reduce the risk of cesarean delivery.
Valtrex Use During Breastfeeding
- Valacyclovir and its active metabolite acyclovir are excreted into human breast milk.
- The amount of acyclovir in breast milk is generally low and would not be expected to cause adverse effects in breastfed infants.
- Caution is still warranted, as the effects of long-term exposure in breastfed infants are unknown. Monitoring the breastfed infant for potential adverse effects is recommended.
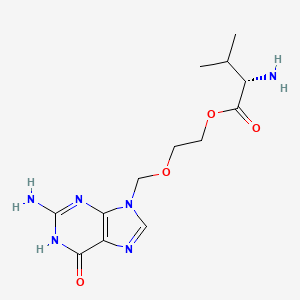
Chemical Structure of Valtrex (Valacyclovir)
The chemical structure of Valtrex (valacyclovir hydrochloride) is:
C13H20N6O4\cdot HCl}$$Thestructuralformulais:L-valine,2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethylester,monohydrochlorideValacyclovirhydrochlorideisawhitetooff-whitepowderwithamolecularweightof360.80.Themaximumsolubilityinwaterat25°Cis174mg/mL.Thekeystructuralfeaturesare:-TheL-valylestergroupattachedtotheacyclovirmoiety-The2-amino-1,6-dihydro-6-oxo-9H-purin-9-ylgroup-
Dosage
Adult Dosing
- Cold Sores (Herpes Labialis): 2 g taken twice daily for 1 day
- Genital Herpes:
- Initial episode: 1 g twice daily for 10 days
- Recurrent episodes: 500 mg twice daily for 3 days
- Suppressive therapy (immunocompetent): 1 g once daily or 500 mg once daily
- Suppressive therapy (HIV-1 infected): 500 mg twice daily
- Reduction of transmission: 500 mg once daily
- Herpes Zoster: 1 g three times daily for 7 days
Pediatric Dosing
- Cold Sores (ages ≥12 years): 2 g twice daily for 1 day
- Chickenpox (ages 2 to <18 years): 20 mg/kg three times daily for 5 days, not to exceed 1 g three times daily
Dosage may need to be adjusted in patients with renal impairment.
Storage
- Store at room temperature between 59°F and 77°F (15°C and 25°C)
- Keep away from light and moisture
- Do not store in the bathroom
Reviews
- Valtrex is generally well-tolerated, with common side effects including headache, nausea, and abdominal pain
- It is effective for treating and suppressing genital herpes, cold sores, and shingles in immunocompetent patients
- Some patients report faster healing of outbreaks and reduced symptom duration when taking Valtrex
- A small percentage of patients may experience more severe side effects like kidney problems, especially those with pre-existing renal impairment
- Overall, Valtrex is considered a safe and effective antiviral medication when used as directed and with appropriate monitoring in patients with risk factors






Reviews
There are no reviews yet.