Zoladex Injection 3.6mg
Zoladex 3.6 mg Implant is a medication used to treat various conditions in both men and women:For Men:
- Prostate cancer – Zoladex can treat prostate cancer, but it is not a cure and does not work for all patients.
For Women:
- Breast cancer – Zoladex is used to treat hormone-sensitive breast cancer in premenopausal women. It can help control the cancer for a period of time, but is not a cure.
- Endometriosis – Zoladex may be used as an alternative to surgery to treat endometriosis.
- Uterine fibroids – Zoladex can be used to treat uterine fibroids.
- Infertility – Zoladex can be used in combination with another medication called gonadotrophin to treat infertility.
- Thinning of the uterus lining – Zoladex can be used to thin the lining of the uterus prior to surgery.
The Zoladex 3.6 mg Implant is injected under the skin every 4 weeks (28 days) by a healthcare provider. It slowly releases the active ingredient goserelin over that time period. Potential side effects include hot flashes, decreased sexual function, injection site reactions, and others.
>IMPORTENT NOTE… Those customers who belongs to lahore avail the opportunity of cash on delivery(COD) with in 30mints.
> Available on cheap price in Pakistan.
> ALL ALTERNATIVES BRANDS ARE AVAILABLE
>ORIGNAL & IMPORTED
> For maore details & price kindly contact on whatsapp.
Original price was: ₨14,500.00.₨13,500.00Current price is: ₨13,500.00.
Description
Zoladex 3.6 mg Implant is a medication used to treat various conditions in both men and women:For Men:
- Prostate cancer – Zoladex can treat prostate cancer, but it is not a cure and does not work for all patients.
For Women:
- Breast cancer – Zoladex is used to treat hormone-sensitive breast cancer in premenopausal women. It can help control the cancer for a period of time, but is not a cure.
- Endometriosis – Zoladex may be used as an alternative to surgery to treat endometriosis.
- Uterine fibroids – Zoladex can be used to treat uterine fibroids.
- Infertility – Zoladex can be used in combination with another medication called gonadotrophin to treat infertility.
- Thinning of the uterus lining – Zoladex can be used to thin the lining of the uterus prior to surgery.
The Zoladex 3.6 mg Implant is injected under the skin every 4 weeks (28 days) by a healthcare provider. It slowly releases the active ingredient goserelin over that time period. Potential side effects include hot flashes, decreased sexual function, injection site reactions, and others. 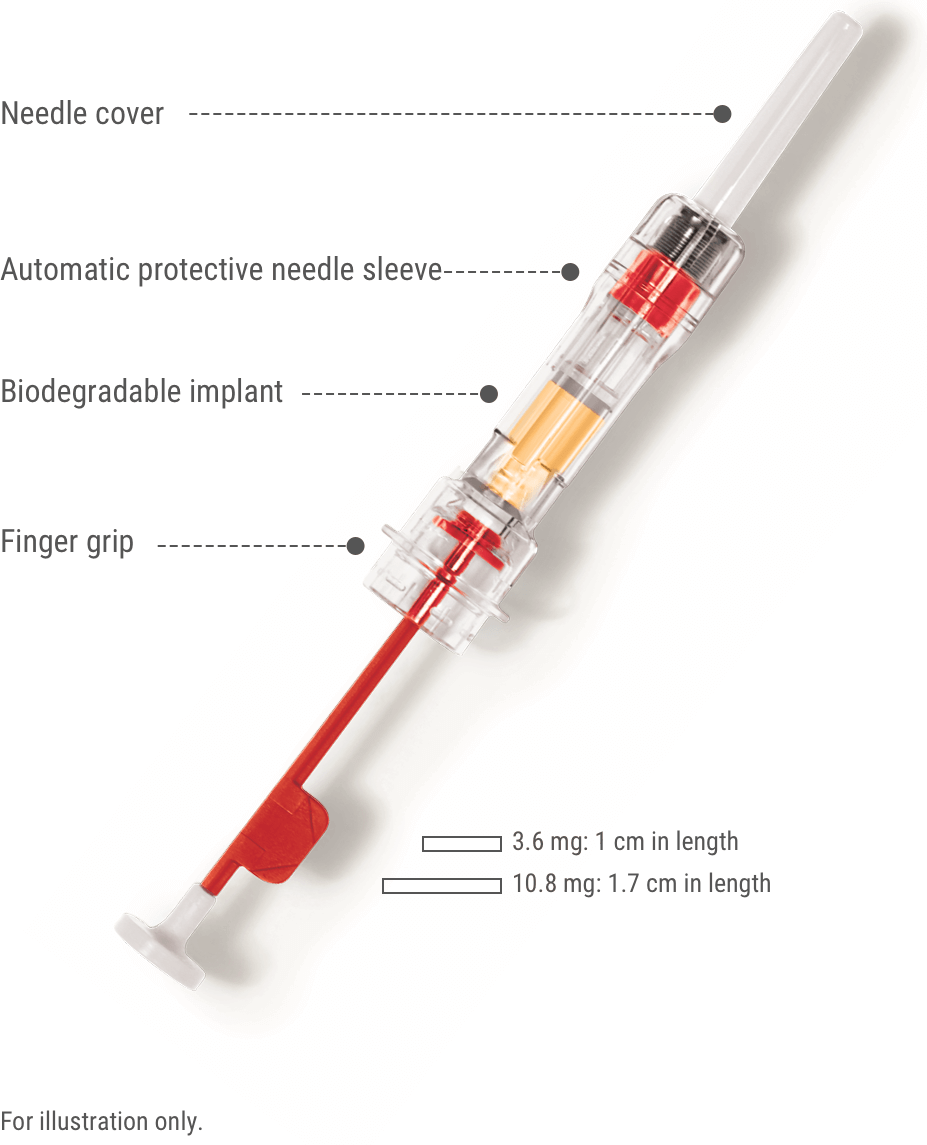
Indications for Men
- Prostate cancer
Indications for Women
- Breast cancer
- Endometriosis
- Uterine fibroids
- Infertility
- Thinning of the uterus lining
Administration
- Injected under the skin every 4 weeks (28 days) by a healthcare provider
- Slowly releases the active ingredient goserelin over that time period
Potential Side Effects
- Hot flashes
- Decreased sexual function
- Injection site reactions
- Others
Important Considerations
- Patients should keep all appointments to receive the Zoladex injections as scheduled
- Missing a dose can cause hormone levels to rise and the condition to worsen
- Patients should discuss any concerns about side effects or stopping treatment with their doctor
Key Benefits of Zoladex 3.6mg:
- Prostate Cancer Treatment: Reduces testosterone levels to treat prostate cancer in men.
- Breast Cancer Treatment: Reduces estrogen levels to treat hormone-sensitive breast cancer in premenopausal women.
- Endometriosis Management: Alleviates symptoms and reduces the size and number of endometrial lesions.
- Uterine Fibroids Treatment: Shrinks fibroids, reduces symptoms, and causes cessation of menses, improving haematological status.
- Endometrial Thinning: Used as a pre-thinning agent before endometrial ablation.
- Assisted Reproduction: Helps regulate and suppress the pituitary gland for assisted reproduction.
Key Ingredients:
- Goserelin Acetate: The active ingredient responsible for reducing hormone levels.
- Lactide/Glycolide Copolymer: An inactive substance used as a carrier for the active ingredient.
Administration and Dosage:
- Men: One 3.6 mg implant injected subcutaneously every 28 days.
- Women: One 3.6 mg implant injected subcutaneously every 28 days, with dosage adjusted based on serum estradiol concentration.
Side Effects:
- Common: Depression, mood swings, sexual impairment, abnormal blood pressure, hot flushes, pimples, increased sweating, hair loss, vaginal dryness, erectile dysfunction, breast enlargement, reaction at the injection site, impaired glucose tolerance, spinal cord compression, paresthesia, headache, arthralgia, bone pain, weight gain, and loss of bone density.
- Uncommon: Hypersensitivity reactions, arthritis, ureteral obstruction, breast tenderness, hypercalcemia.
- Rare: Ovarian cysts, pituitary hemorrhage, pituitary tumor, psychosis.
Precautions and Contraindications:
- Contraindicated: In patients with a history of sensitivity to LHRH or any excipients, pregnant or breastfeeding women.
- Precautions: Use with caution in patients with low BMI, renal impairment, or full anticoagulation medication.
Important Notes:
- Dosage Adjustment: No dosage adjustment is necessary for patients with hepatic or renal impairment, the elderly, or children.
- Duration of Treatment: Treatment duration varies depending on the condition, with a maximum of six months for benign gynaecological conditions.
- Monitoring: Patients should be monitored for potential side effects and bone mineral loss. Zoladex 3.6 mg is administered via subcutaneous injection every 28 days:
- The patient is placed in a comfortable position with the upper body slightly raised. An area of the anterior abdominal wall below and two inches out from the navel line is prepared with an alcohol swab.
- The foil pouch containing the syringe is examined for damage. The syringe is removed and held at a slight angle to the light to confirm at least part of the Zoladex implant is visible.
- The red plastic safety tab is grasped and pulled away from the syringe, then discarded. The needle cover is removed.
- Holding the syringe around the protective sleeve and using an aseptic technique, the patient’s skin is pinched. With the bevel of the needle facing up, it is inserted at a 30 to 45 degree angle until the protective sleeve touches the skin.
- The plunger is depressed until it cannot be depressed further, administering the Zoladex implant and activating the protective sleeve.
- The needle is withdrawn and the patient is monitored for signs or symptoms of abdominal hemorrhage.
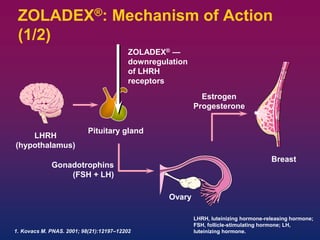
Zoladex 3.6 mg works by inhibiting pituitary gonadotropin secretion, leading to suppression of testosterone levels in men and estrogen levels in women.The mechanism of action is as follows:
- Zoladex contains the active ingredient goserelin, which is a synthetic analogue of the natural hormone gonadotropin-releasing hormone (GnRH).
- When administered, Zoladex acts on the pituitary gland to initially increase the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
- This initial stimulation leads to a temporary rise in testosterone levels in men and estrogen levels in women.
- With chronic administration, Zoladex causes sustained suppression of pituitary gonadotropins, leading to a decrease in testosterone levels in men and estrogen levels in women to the range normally seen in surgically castrated individuals.
- The suppression of sex hormones leads to regression or inhibition of growth of hormone-sensitive tumors, such as prostate cancer in men and hormone-sensitive breast cancer in premenopausal women.
- In animal and in vitro studies, administration of goserelin resulted in the regression or inhibition of growth of the hormone-sensitive dimethylbenzanthracene (DMBA)-induced rat mammary tumor and Dunning R3327 prostate tumor.
 GoserelinMedication used to suppress sex hormone production in cancer treatmentMoreTrade NamesZoladex, othersDrug ClassGnRH analogue, GnRH agonist, AntigonadotropinMedical UseTreatment of breast and prostate cancer, endometriosis, uterine fibroids
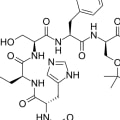
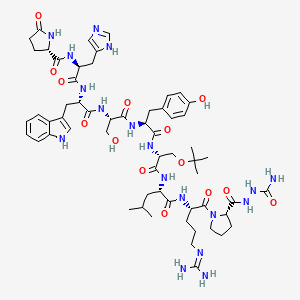
GoserelinMedication used to suppress sex hormone production in cancer treatmentMoreTrade NamesZoladex, othersDrug ClassGnRH analogue, GnRH agonist, AntigonadotropinMedical UseTreatment of breast and prostate cancer, endometriosis, uterine fibroidsThe chemical structure of Zoladex 3.6 mg is represented by the molecular formula C59H84N18O14. This formula indicates that the molecule contains 59 carbon atoms, 84 hydrogen atoms, 18 nitrogen atoms, and 14 oxygen atoms.Here is a detailed breakdown of the chemical structure:
- Pyro-Glu-His-Trp-Ser-Tyr-D-Ser(But)-Leu-Arg-Pro-Azgly-NH2: This is the amino acid sequence of goserelin, the active ingredient in Zoladex. It is a synthetic analogue of gonadotropin-releasing hormone (GnRH).
- Acetate: The acetate group is a functional group that is added to the goserelin molecule to enhance its stability and solubility. This modification helps the molecule to be more easily administered and absorbed by the body.
Storage
- Zoladex 3.6 mg Implant should be stored below 25°C .
- The implant should be kept in the original package until it is administered by a healthcare provider. Taking it out of the package may dislodge the pellet .
- It should be kept in a cool, dry place away from heat, moisture, and reach of children .
- Do not store it in the bathroom or leave it in the car on hot days .
Reviews
The search results do not contain any direct reviews or feedback from patients about using Zoladex 3.6 mg Implant. The information provided is focused on the technical details, administration, and storage of the medication.
Zoladex 3.6 mg helps manage endometriosis symptoms in the following ways:
Reduces Estrogen Levels
Zoladex contains the active ingredient goserelin, which is a synthetic analogue of gonadotropin-releasing hormone (GnRH). It acts on the pituitary gland to suppress the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to a decrease in estrogen levels produced by the ovaries.
Shrinks Endometrial Lesions
By reducing estrogen levels, Zoladex causes regression of endometrial lesions (implants) outside the uterus. In clinical studies, 62-63% of patients had a ≥50% reduction in the extent of endometrial lesions after 6 months of Zoladex treatment.
Relieves Pelvic Pain
Zoladex significantly reduces the clinical symptoms of endometriosis, including pelvic pain. Within 4 weeks of starting treatment, symptoms were reduced by about 84% on average.
Induces Amenorrhea
Zoladex leads to amenorrhea (absence of menstrual periods) in 80-92% of women within 8 weeks of starting treatment. Menses usually resumes within 8 weeks after completing 6 months of therapy.The clinical significance of shrinking endometrial lesions is not fully known, and laparoscopic staging of endometriosis does not always correlate with symptom severity. However, Zoladex provides effective relief of pelvic pain and other symptoms of endometriosis for the duration of treatment.
how quickly you can expect relief from symptoms after starting Zoladex 3.6 mg:
Onset of Symptom Relief
- It takes 2-4 weeks after the first Zoladex 3.6 mg injection for hormone levels (testosterone or estrogen) to start decreasing.
- Many of Zoladex’s side effects, such as hot flashes, acne, and sexual problems, are related to this drop in hormone levels. As a result, patients may notice most of Zoladex’s side effects starting within 2-4 weeks of the first injection.
Endometriosis Symptom Relief
- For patients with endometriosis, Zoladex 3.6 mg can provide significant relief of pelvic pain and other symptoms within 4 weeks of starting treatment.
- Clinical studies show that Zoladex reduced endometriosis symptoms by about 84% on average within the first 4 weeks.
Prostate Cancer Symptom Relief
- For prostate cancer patients, Zoladex 3.6 mg can lead to a temporary worsening of symptoms, such as increased bone pain, in the first few weeks after starting treatment.
- This is due to an initial surge in testosterone levels before the medication suppresses them.
Breast Cancer Symptom Relief
- Breast cancer patients may also experience a worsening of symptoms, such as increased pain or tumor size, in the first few weeks after starting Zoladex 3.6 mg.
- These effects are usually short-lived and resolve as the medication takes effect. Zoladex 3.6 mg can cause the following immediate changes in hormone levels:
In Men:
- Initial Increase in Testosterone Levels
- Following the first administration of Zoladex 3.6 mg in men, there is an initial increase in serum luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels.
- This leads to a subsequent temporary increase in serum testosterone levels.
In Women:
- Decrease in Estrogen Levels
- Within 3 weeks of the first Zoladex 3.6 mg injection in women, serum estradiol levels are suppressed to postmenopausal levels.
- Serum LH and FSH levels are also suppressed to follicular phase levels within 4 weeks.
Temporary Worsening of Symptoms
- The initial surge in hormone levels before suppression can lead to a temporary worsening of symptoms in some patients:
- For prostate cancer patients, there may be increased bone pain in the first few weeks.
- Breast cancer patients may experience increased pain or tumor size initially.
However, with continued Zoladex 3.6 mg treatment, the sustained suppression of pituitary gonadotropins and sex hormone levels is achieved within 2-4 weeks. This leads to the desired therapeutic effects for conditions like prostate cancer, breast cancer, endometriosis, and uterine fibroids.Patients should be monitored closely during the initial weeks of Zoladex 3.6 mg therapy and report any worsening of symptoms to their healthcare provider.
- Initial Increase in Testosterone Levels







Reviews
There are no reviews yet.