Carfilzomib 60’mg Injection
Carfilzomib 60 mg Injection
- Medication used to treat multiple myeloma, a type of cancer
- Works by stopping the growth of cancer cells
- Given as an intravenous (IV) injection, usually every 1-2 weeks
- Common side effects include anemia, diarrhea, fatigue, and low blood pressure
- Patients need to be monitored closely for serious side effects
- Used alone or with other cancer medications for patients who have tried other treatments
Carfilzomib Uses
Carfilzomib 60 mg injection is used to treat multiple myeloma, a type of cancer that affects the bone marrow. It is given:
- Alone or with other cancer medications
- For patients whose myeloma has returned or stopped responding to other treatments
- As an intravenous (IV) injection, usually weekly
Carfilzomib works by stopping the growth and killing multiple myeloma cancer cells. It is an approved treatment for adults with relapsed or refractory multiple myeloma.
How Carfilzomib is Given
- Carfilzomib is given as an intravenous (IV) infusion
- The infusion is given over 10-30 minutes
- Typical dosing is 20/27 mg/m2 or 20/56 mg/m2 twice weekly
- It is given on days 1, 2, 8, 9, 15, and 16 of a 28-day treatment cycle
- Carfilzomib can be given alone or with other multiple myeloma medications
The key points are that carfilzomib is administered intravenously over 10-30 minutes, typically twice a week, as part of a treatment regimen for multiple myeloma.
 CarfilzomibAnti-cancer medication
CarfilzomibAnti-cancer medication
Description
Carfilzomib 60 mg Injection
- Medication used to treat multiple myeloma, a type of cancer
- Works by stopping the growth of cancer cells
- Given as an intravenous (IV) injection, usually every 1-2 weeks
- Common side effects include anemia, diarrhea, fatigue, and low blood pressure
- Patients need to be monitored closely for serious side effects
- Used alone or with other cancer medications for patients who have tried other treatments
Carfilzomib Uses
Carfilzomib 60 mg injection is used to treat multiple myeloma, a type of cancer that affects the bone marrow. It is given:
- Alone or with other cancer medications
- For patients whose myeloma has returned or stopped responding to other treatments
- As an intravenous (IV) injection, usually weekly
Carfilzomib works by stopping the growth and killing multiple myeloma cancer cells. It is an approved treatment for adults with relapsed or refractory multiple myeloma.
How Carfilzomib is Given
- Carfilzomib is given as an intravenous (IV) infusion
- The infusion is given over 10-30 minutes
- Typical dosing is 20/27 mg/m2 or 20/56 mg/m2 twice weekly
- It is given on days 1, 2, 8, 9, 15, and 16 of a 28-day treatment cycle
- Carfilzomib can be given alone or with other multiple myeloma medications
The key points are that carfilzomib is administered intravenously over 10-30 minutes, typically twice a week, as part of a treatment regimen for multiple myeloma.
 CarfilzomibAnti-cancer medicationMoreDefinitionCarfilzomib is a selective proteasome inhibitor used as an anti-cancer medication.Trade NameKyprolisFDA Approval DateJuly 2012
CarfilzomibAnti-cancer medicationMoreDefinitionCarfilzomib is a selective proteasome inhibitor used as an anti-cancer medication.Trade NameKyprolisFDA Approval DateJuly 2012Based on the search results, the structure of carfilzomib contributes to its function as a proteasome inhibitor in the following ways:
Structural Features of Carfilzomib
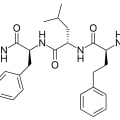
- Carfilzomib is a modified tetrapeptide that contains an epoxyketone pharmacophore.
- The peptide portion is responsible for selective binding to the proteasome pocket.
- The epoxyketone group is a key structural feature that allows carfilzomib to irreversibly bind to and inhibit the proteasome.
Mechanism of Action
- Carfilzomib irreversibly binds to and inhibits the chymotrypsin-like activity of the 20S proteasome, the proteolytic core of the 26S proteasome.
- The epoxyketone group of carfilzomib covalently and selectively binds to the N-terminal threonine residues in the active sites of the proteasome.
- This inhibition of proteasome-mediated protein degradation leads to the accumulation of polyubiquitinated proteins, causing cell cycle arrest and apoptosis in cancer cells.
Selectivity
- Compared to the proteasome inhibitor bortezomib, carfilzomib has a larger and more lipophilic chemical structure that allows for more selective binding to the proteasome.
- This improved selectivity for the proteasome contributes to the better safety profile of carfilzomib compared to earlier proteasome inhibitors.
Active Ingredient
- Carfilzomib
Inactive Ingredients (Kyprolis brand)
- Betadex sulfobutyl ether sodium
- Anhydrous citric acid
- Sodium hydroxide
- Water for injection
The active ingredient, carfilzomib, is a synthetic tetrapeptide proteasome inhibitor with the molecular formula C40H57N5O7 and a molecular weight of 719.9.
Dosage
- 20/27 mg/m2 or 20/56 mg/m2 given twice weekly as an IV infusion over 10-30 minutes
- Administered on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle
Storage
- Store unopened vials refrigerated at 2-8°C (36-46°F)
- Keep vials in original package to protect from light
- Reconstituted solution can be stored refrigerated for up to 24 hours
Reviews
- Effective for treating relapsed or refractory multiple myeloma
- Common side effects include anemia, fatigue, high blood pressure, low platelets
- Careful monitoring and management of side effects is important during treatment
Order siteVitamins collection.comContact0307-7532291/0320-6421093






Reviews
There are no reviews yet.