Kadcyla (Trastuzumab Emtansine) 160mg Imported Available In Pakistan
Kadcyla, known generically as trastuzumab emtansine, is a targeted cancer therapy primarily used for the treatment of HER2-positive breast cancer. In Pakistan, it is available in an injectable form with a dosage of 160 mg (20 mg/ml), manufactured by Roche Pakistan Pvt Ltd.
Product Details
- Form: Injection
- Pack Size: 1 vial
- Manufacturer: Roche Pakistan Pvt Ltd
- Generic Category: Antineoplastic Monoclonal Antibodies
- Active Ingredient: Ado-Trastuzumab Emtansine
Original price was: ₨45,000.00.₨39,999.00Current price is: ₨39,999.00.
Description
Kadcyla, known generically as trastuzumab emtansine, is a targeted cancer therapy primarily used for the treatment of HER2-positive breast cancer. In Pakistan, it is available in an injectable form with a dosage of 160 mg (20 mg/ml), manufactured by Roche Pakistan Pvt Ltd.
Product Details
- Form: Injection
- Pack Size: 1 vial
- Manufacturer: Roche Pakistan Pvt Ltd
- Generic Category: Antineoplastic Monoclonal Antibodies
- Active Ingredient: Ado-Trastuzumab Emtansine
-
Key Benefits
- Targeted Therapy: Kadcyla combines the HER2-targeting properties of trastuzumab with a chemotherapy agent, allowing for precise treatment of HER2-positive cancer cells while minimizing damage to healthy tissues
- Indications: It is indicated for patients with HER2-positive metastatic breast cancer who have previously received trastuzumab and taxane-based chemotherapy. Additionally, it is used as adjuvant therapy for early breast cancer patients with residual invasive disease after neoadjuvant treatment
- Improved Efficacy: Clinical studies have shown that Kadcyla can improve survival rates and reduce the risk of disease progression compared to standard treatments in eligible patients
- Convenient Administration: Administered as an intravenous infusion, Kadcyla allows for controlled dosing under medical supervision, which can be beneficial for managing potential side effects
Key Ingredients
- Trastuzumab Emtansine: The active ingredient in Kadcyla is trastuzumab emtansine, an antibody-drug conjugate that consists of:
- Trastuzumab: A humanized IgG1 monoclonal antibody that specifically targets the HER2 receptor on cancer cells.
- DM1 (Maytansine derivative): A potent microtubule inhibitor that interferes with cell division, linked to trastuzumab via a stable linker (MCC) to form the complete conjugate
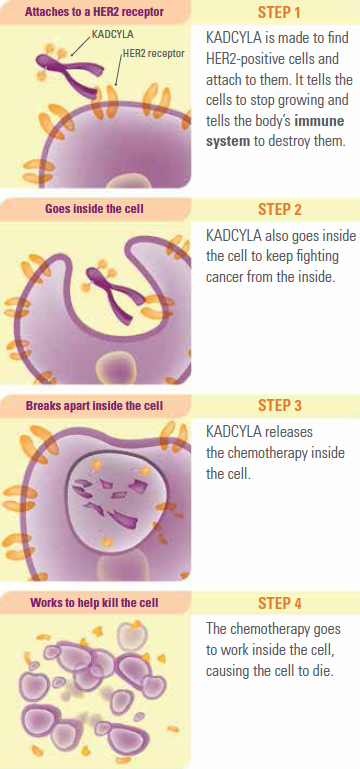
- Kadcyla (ado-trastuzumab emtansine) is an innovative cancer treatment designed specifically for HER2-positive breast cancer. Its mechanism of action involves a combination of targeted therapy and chemotherapy, allowing it to effectively target and kill cancer cells. Here’s how it works:
Mechanism of Action
- Targeting HER2 Receptors: Kadcyla utilizes trastuzumab, a monoclonal antibody that specifically binds to the HER2 receptor, which is overexpressed on the surface of HER2-positive breast cancer cells. By attaching to these receptors, Kadcyla blocks the signals that promote cell growth and division, thereby inhibiting tumor growth
- Internalization and Drug Release: Once Kadcyla binds to the HER2-positive cancer cell, the entire complex is internalized through a process called endocytosis. Inside the cell, Kadcyla is broken down, releasing its cytotoxic component, DM1 (a derivative of maytansine)
- Cytotoxic Action: DM1 disrupts microtubule function, which is essential for cell division. By preventing microtubule polymerization, DM1 causes cell cycle arrest and ultimately leads to apoptosis (programmed cell death) in the cancer cells
- Reduced Damage to Healthy Cells: The design of Kadcyla as an antibody-drug conjugate allows it to deliver chemotherapy directly to the cancer cells while minimizing exposure to healthy tissues. This targeted delivery helps reduce side effects compared to traditional chemotherapy
-
Combination with Other Treatments
- Prior Therapies: Kadcyla is designed for patients who have previously received trastuzumab and a taxane, either separately or in combination. This means that it is often used after these treatments have been administered, rather than concurrently with them
- Adjuvant Setting: In the adjuvant treatment of early breast cancer, Kadcyla is used following neoadjuvant therapy that includes trastuzumab and taxanes. This indicates that while it is not typically combined with these therapies during administration, it follows them in the treatment sequence
- Clinical Trials: Some studies may explore Kadcyla in combination with other agents, particularly in clinical trial settings. However, any such combinations would need to be supported by clinical evidence demonstrating safety and efficacy.
- Consultation with Healthcare Providers: It is crucial for patients to discuss their overall treatment plan with their oncologist or healthcare provider. They can provide guidance on whether Kadcyla can be safely combined with other treatments based on individual health status and treatment goals.
-
Key Precautions
- Cardiac Monitoring: Kadcyla can cause cardiac toxicity, including reductions in left ventricular ejection fraction (LVEF). Patients should have their heart function assessed before starting treatment and monitored regularly throughout therapy. Any significant decline in heart function may require dose adjustments or discontinuation of the drug
- Hepatic Function: Hepatotoxicity, including liver failure, has been reported in patients treated with Kadcyla. Liver function tests (LFTs) should be conducted before initiation and prior to each dose. If there are significant elevations in liver enzymes, dose modifications or discontinuation may be necessary
- Infusion Reactions: Patients may experience infusion-related reactions during administration. Monitoring for symptoms during and after infusion is essential, and any severe reactions may require slowing or stopping the infusion
- Pregnancy and Contraception: Kadcyla can cause harm to the fetus if administered during pregnancy. Effective contraception is advised for both male and female patients during treatment and for at least 7 months after the last dose. Breastfeeding is also not recommended during treatment
- Allergic Reactions: Patients should inform their healthcare provider of any history of allergic reactions to Kadcyla or similar medications, as hypersensitivity reactions can occur
- Drug Interactions: Certain medications may interact with Kadcyla, including some antifungals, antibiotics, and medications for hepatitis or depression. Patients should disclose all medications they are taking to their healthcare provider
Dietary Restrictions
While specific dietary restrictions are not extensively detailed for Kadcyla, general recommendations include:
- Hydration: Maintaining adequate hydration can help manage side effects such as nausea and vomiting.
- Balanced Diet: A well-balanced diet rich in fruits, vegetables, lean proteins, and whole grains can support overall health during treatment.
- Alcohol Consumption: It is advisable to limit or avoid alcohol intake, as it may exacerbate liver toxicity and interfere with overall health during cancer treatment.
-
Foods and Drinks to Avoid
- Sugary Foods:
- High sugar intake can promote inflammation and potentially accelerate cancer growth. Avoid foods with added sugars, such as:
- Sodas
- Fruit juices
- Baked goods made with white flour
- Candy and desserts
- High sugar intake can promote inflammation and potentially accelerate cancer growth. Avoid foods with added sugars, such as:
- Saturated and Trans Fats:
- Diets high in saturated fats may contribute to the growth of breast cancer cells. Limit foods such as:
- Fried foods
- Processed snacks (e.g., chips, cookies)
- Margarine and shortening
- Fatty cuts of red meat
- Diets high in saturated fats may contribute to the growth of breast cancer cells. Limit foods such as:
- Alcohol:
- Alcohol consumption can exacerbate liver toxicity and may interfere with treatment efficacy. It is advisable to limit or avoid alcohol while on Kadcyla.
- Processed Meats:
- High consumption of processed meats has been linked to an increased risk of breast cancer. Limit or avoid:
- Sausages
- Hot dogs
- Bacon
- High consumption of processed meats has been linked to an increased risk of breast cancer. Limit or avoid:
- High-Sodium Foods:
- Some patients may experience fluid retention, so it is wise to limit high-sodium foods, which can exacerbate this issue, including:
- Canned soups
- Processed meals
- Salty snacks
- Some patients may experience fluid retention, so it is wise to limit high-sodium foods, which can exacerbate this issue, including:
- Caffeine:
- While moderate caffeine consumption is generally acceptable, excessive amounts can lead to dehydration and may worsen side effects like anxiety or insomnia.
General Recommendations
- Hydration: Maintain adequate hydration by drinking plenty of water.
- Balanced Diet: Focus on a diet rich in fruits, vegetables, whole grains, and lean proteins to support overall health.
- Small Meals: Eating several small meals throughout the day may help manage nausea and other gastrointestinal side effects.
-
Best Foods to Eat While on Kadcyla
1. Fruits and Vegetables
- Berries: Blueberries, blackberries, and strawberries are rich in antioxidants and vitamins that can support overall health.
- Cruciferous Vegetables: Broccoli, cauliflower, kale, and Brussels sprouts contain compounds that may help reduce cancer cell growth.
- Leafy Greens: Spinach, arugula, and collard greens are nutrient-dense and provide essential vitamins and minerals.
- Citrus Fruits: Oranges, lemons, and grapefruits (in moderation) are high in vitamin C, which can boost immune function.
2. Healthy Fats
- Olive Oil: Extra-virgin olive oil is a source of healthy monounsaturated fats and has anti-inflammatory properties.
- Avocados: Rich in healthy fats and fiber, avocados can help maintain energy levels and support heart health.
- Nuts and Seeds: Walnuts, flaxseeds, and chia seeds provide omega-3 fatty acids, which are beneficial for reducing inflammation.
3. Lean Proteins
- Fish: Fatty fish like salmon, mackerel, and sardines are high in omega-3 fatty acids and protein.
- Poultry: Skinless chicken or turkey can provide lean protein necessary for maintaining muscle mass.
- Legumes: Beans, lentils, and chickpeas are excellent plant-based protein sources that also provide fiber.
4. Whole Grains
- Brown Rice: A good source of complex carbohydrates that provide sustained energy.
- Quinoa: High in protein and gluten-free, quinoa is a nutritious grain option.
- Oats: Oatmeal is gentle on the stomach and provides fiber that aids digestion.
5. Hydration
- Water: Staying well-hydrated is crucial during treatment. Aim for adequate fluid intake throughout the day.
- Herbal Teas: Non-caffeinated herbal teas can be soothing and hydrating.
Foods to Consider for Enhanced Effectiveness
Some studies suggest that certain foods may amplify the effects of treatments like Kadcyla:
- Turmeric: Contains curcumin, which has anti-inflammatory properties.
- Green Tea: Rich in antioxidants that may support overall health.
- Storage Conditions: Kadcyla should be stored at 2°C to 8°C (36°F to 46°F) and protected from light. It must not be frozen. Once reconstituted, the solution can be stored at room temperature for a limited time but should be used promptly.
- Shelf Life: The lyophilized powder form has a specific expiration date that should be adhered to, ensuring that it is not used beyond this date
- Recommended Dose: The standard dosage of Kadcyla is 3.6 mg/kg of body weight, administered as an intravenous infusion every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity occurs. This dosage is applicable for both early-stage and metastatic breast cancer.
- Administration: The first infusion should be given over 90 minutes, with subsequent infusions potentially shortened to 30 minutes if the patient tolerated the previous doses well. Patients should be monitored during and after the infusion for any adverse reactions, especially during the initial dose
Dosage Adjustments
- If patients experience side effects such as infusion-related reactions, liver toxicity, or decreased heart function, their dosage may need to be adjusted. Specific guidelines exist for managing these conditions, including potential dose reductions or treatment interruptions
Reviews
- Patient Feedback: Reviews of Kadcyla generally indicate that many patients appreciate its targeted approach to treating HER2-positive breast cancer. Patients often report positive experiences regarding its efficacy in managing their condition.
- Side Effects: Common side effects noted in reviews include fatigue, nausea, and infusion-related reactions. However, many patients find that these side effects are manageable with appropriate medical support.
- Overall Satisfaction: Many patients express satisfaction with their treatment outcomes when using Kadcyla, particularly in terms of disease control and quality of life improvements during therapy
-
Storage
- Storage Conditions: Kadcyla should be stored at 2°C to 8°C (36°F to 46°F) and protected from light. It must not be frozen. Once reconstituted, the solution can be stored at room temperature for a limited time but should be used promptly.
- Shelf Life: The lyophilized powder form has a specific expiration date that should be adhered to, ensuring that it is not used beyond this date
- Sugary Foods:







Reviews
There are no reviews yet.